A3. Responsible Conduct of Research and Research Ethics in Finland
-
A3. Responsible Conduct of Research and Research Ethics in Finland
-
A3. Responsible Conduct of Research and Research Ethics in Finland
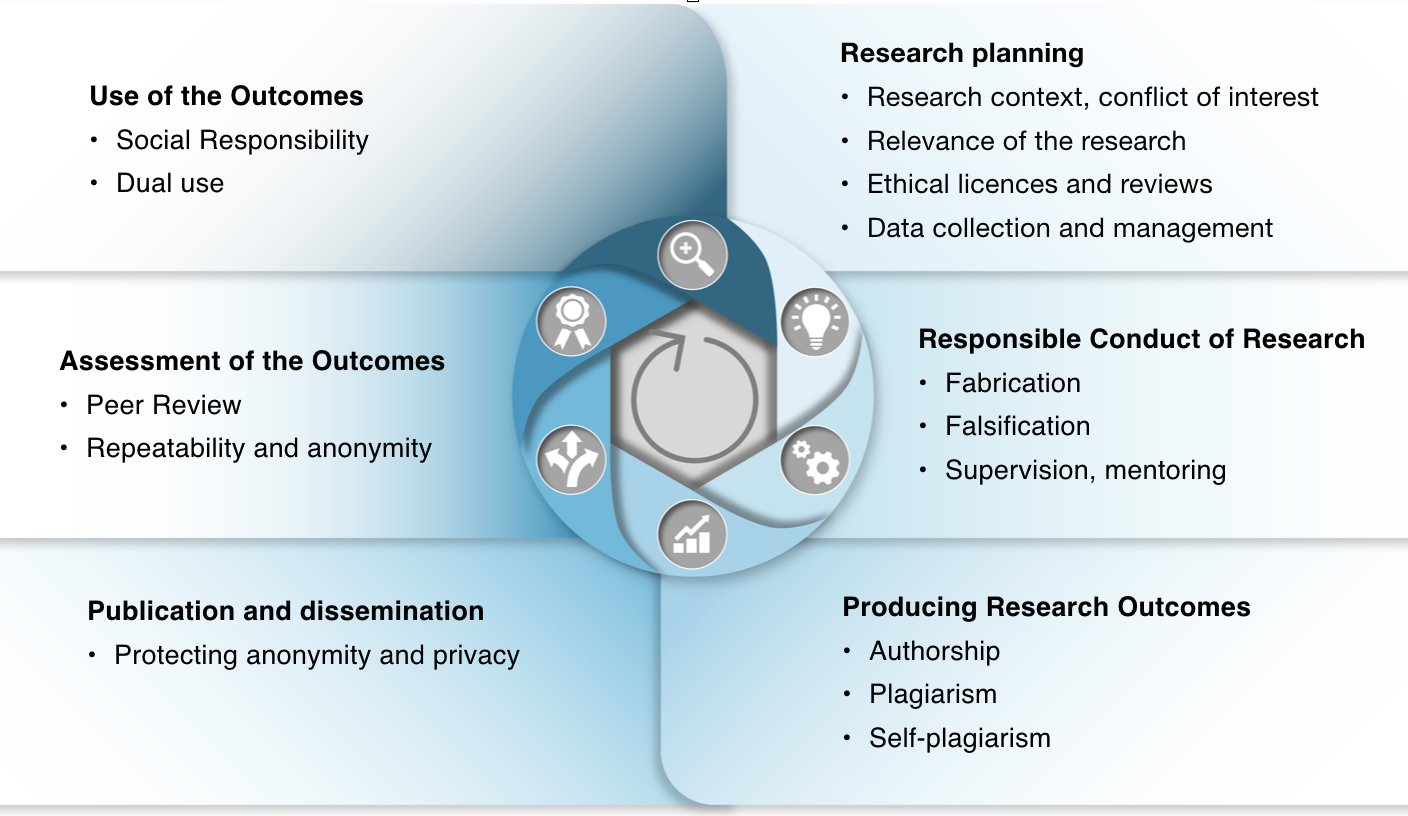
This course introduces the responsible conduct of research (RCR) and its violations, according to the RCR guideline of the Finnish National Board on Research Integrity (TENK). TENK's RCR guideline is part of the ethical self-regulation of the research community and it is applied to all scientific, artistic and other research activities, RDI projects and lifecycle procedures in the organisations committed to the guideline. In Finland, all universities and other research institutions are committed to the guideline. The newest, updated version of the guideline was launched in early 2023 and all references in the course material are made to this version.
TENK has supplemented the guideline on responsible conduct of research with recommendations and guides. These include a template for researchers’ curriculum vitae and a recommendation for agreeing on authorship. International research integrity guidelines include the European Code of Conduct for Research Integrity for projects that have received EU funding, the Code of Conduct and Best Practice Guidelines for Journal Editors for Finnish COPE members and the so-called Vancouver style, i.e. the Uniform Requirements for Manuscripts Submitted to Biomedical Journals. These other guidelines are introduced more in detail in the following sections of this course.
TENK's RCR guideline is in accordance with international guidelines, but it also provides instructions for defining RCR violations and investigating alleged violations in Finland. Unlike TENK's RCR guideline, international guidelines are not similarly binding on the Finnish research community and organisations that fund research.
Some fields in Finland have specific bodies for research integrity, such as the National Advisory Board on Social Welfare and Health Care Ethics (ETENE), the National Committee on Medical Research Ethics (Tukija) and the Advisory Board on Biotechnology (BTNK). The norms approved by these bodies offer more detailed professional ethics guidelines on matters such as the relationship between the researcher and the research participant or research subject.
There are also regional, discipline-specific or organisation-specific research ethics committees in connection with hospitals, higher education institutions and research institutions. They are tasked to carry out ethical reviews where the research setting of a project is reviewed from the perspective of general and discipline-specific ethical principles before a project is launched. The review assesses the potential harm and damage caused to the subjects, their families or the researcher in relation to the informative value sought by the research. Ethical review processes are discussed more in detail in section B2 Data Collection.
Finnish Advisory Board on Research Integrity
RCR Guideline 2023Basic principles
According to the European Code of Conduct for Research Integrity, the basic principles of research integrity are reliability, honesty, respect and accountability (see previous section).
Responsible conduct of research consists of good research practices that ensure that research integrity is maintained throughout the lifecycle of scientific activities. Good research practices are therefore also part of the quality assurance of organisations belonging in the research community. Failure to comply with them can, at worst, lead to an alleged violation of responsible conduct of research and subsequently the RCR process.
Good scientific practice
In line with the European Code of Conduct for Research Integrity, good research practices can be linked to eight areas of scientific activity: 1) the research environment, 2) training, guidance and mentoring, 3) conducting scientific work, 4) ethics and foresight, 5) research data practices and management, 6) cooperation, 7) authorship, publishing and dissemination, and 8) expert and review tasks.
Following roughly this list of areas, the examples of good research practices and their violations are introduced in the beginning of each section of the course.
Activities that violate responsible conduct of research
Overview, assessment of severity and RCR violation
Acting against the responsible conduct of research violates the basic principles of responsible conduct of research and damages the quality, credibility, authorship or cooperation of scientific activities. The activities may also be unlawful, in which case the related actions are processed in separate official or judicial procedures in addition to the RCR process. Differences of opinion or disagreements related to scientific theories, methods or interpretations of results are part of scientific discourse instead and are generally not in violation of responsible conduct of research.
RCR violations include:
- serious intentional violations of responsible conduct of research; or
- actions that seriously neglect responsible conduct of research due to indifference or negligence in a situation where the practices could have been followed; or
- serious neglect of responsible conduct of research caused by ignorance as a result of not having looked into matters even there had been an opportunity to do so.
In Finland, violations of responsible conduct of research are divided into two categories: research misconduct and disregard for the responsible conduct of research. They can occur in any area and at any stage of scientific activity.
In the guideline and in this course, actions that violate responsible conduct of research are described through definitions and examples. However, the examples do not cover all actions that violate responsible conduct of research. The assessment of whether a case constitutes a violation or responsible conduct of research is determined case by case. That is why it is impossible to have a detailed and unambiguous list of the actions that are interpreted as RCR violations.Process of handling alleged violations of responsible conduct of research i.e. the RCR process
Video: Investigation of research misconduct in Finland
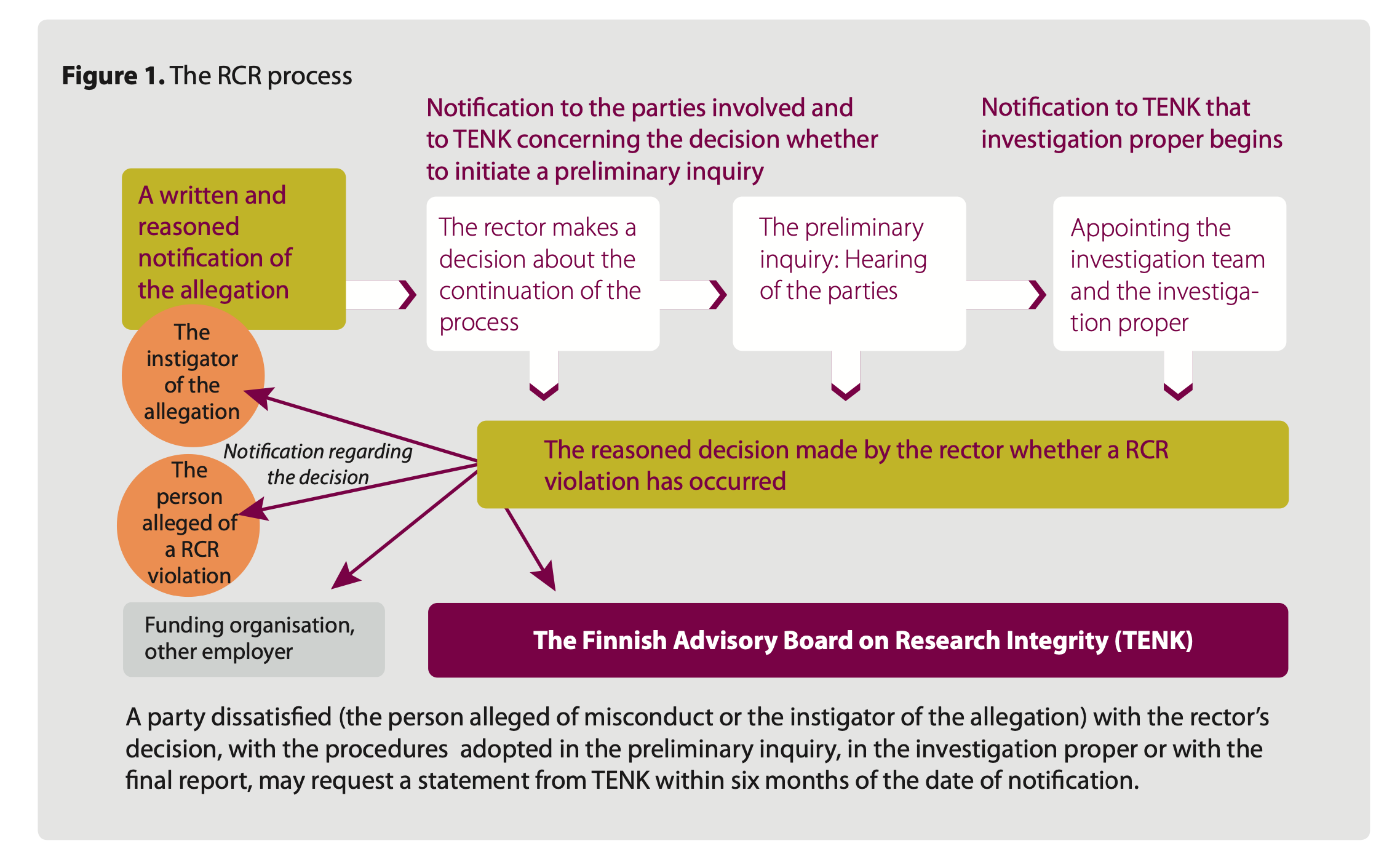
Process steps
The RCR process is carried out by the organisation committed to the RCR guideline where the alleged violations have taken place or are ongoing. There are three steps to the RCR process: 1) written notification and filing, 2) preliminary inquiry and 3) investigation proper (Figure 3). The director of the organisation (the Chancellor at the University of Helsinki) conveys information on the notified allegation and decisions related to the different stages of the process to both the parties involved in the process and the Finnish National Board on Research Integrity TENK so that TENK can monitor compliance with the guideline and the state of research integrity in Finland.
The Research Integrity Advisers
The advisers give advice to researchers and other staff in their organisation. The discussion with an adviser is absolutely confidential. They may be asked for advice, if, for example, there is a suspicion of a violation of responsible conduct of research or if someone has themselves been suspected of a violation.
The advisers are familiar with the responsible conduct of research (RCR) investigation process monitored by TENK and may, for example, provide assistance in drafting an RCR notification. However, they are not lawyers. They are a neutral source of advice, and they can, where necessary, provide advice to both parties in a dispute. The advisers do not participate in the RCR process and they do not give their view on whether misconduct has occurred. That decision is made by the top management of the organisation according to the RCR process.
Research Integrity Advisers at the University of Helsinki are : Heikki Järvinen ja Noora Pyyry (Kumpulan campus), Katarina Pelin (Viikki and Meilahti campuses), Helena Korpelainen (Viikki), Maria Fredriksson-Ahomaa (Viikki), Petri Ylikoski (Centre campus) ja Salla Kurhila (Centre campus).Read more about Research Integrity Advisers from Responsible Research Articles.
Recommendations of the Finnish National Board on Research Integrity TENK for Research Integrity Advisers (PDF) (published 15.2.2018; under revision in 2023)

- serious intentional violations of responsible conduct of research; or
-